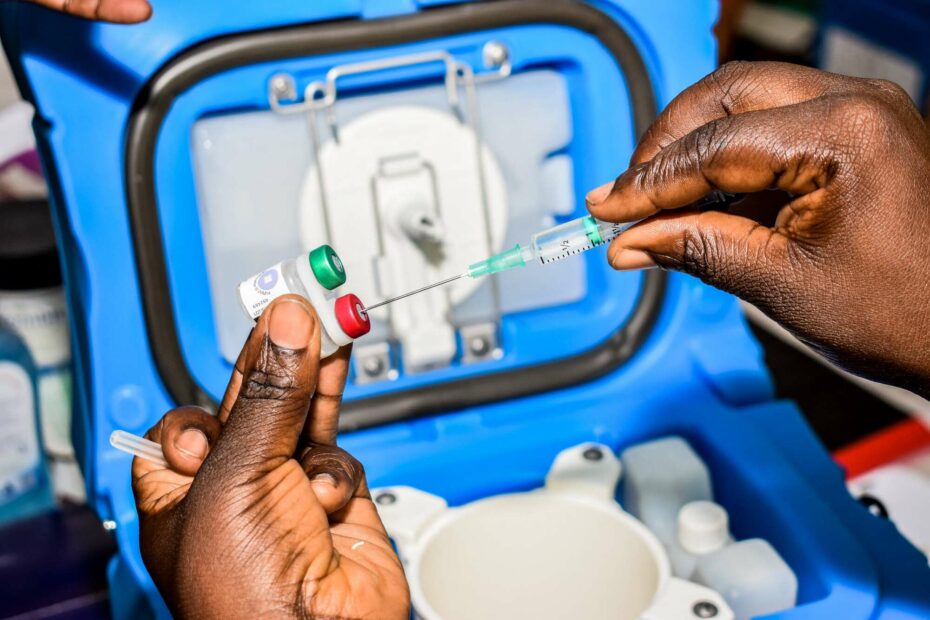
Cameroon Rolls out a World-First Malaria Vaccine. This will contribute to a global breakthrough anticipated to preserve numerous children’s lives throughout Africa. WHO sanctioned the RTS, S vaccine, developed by the British pharmaceutical company GSK. It targets infants residing in the 42 most severely affected districts in Cameroon.
The country will pioneer the incorporation of vaccine doses into a regular immunization program. The intention is to build on the success of pilot initiatives in Kenya, Ghana, and Malawi. The start of the rollout on Monday, is a significant milestone in the prolonged campaign to fight Malaria in Africa. Notably, twenty additional countries intend to introduce the program later this year (Global Vaccine Alliance Gavi).
Note that the four-dose vaccine’s efficacy is approximately 30%. Its protective effects begin to diminish after several months. GSK has indicated a production capacity of around 15 million doses annually.
How will it Work?
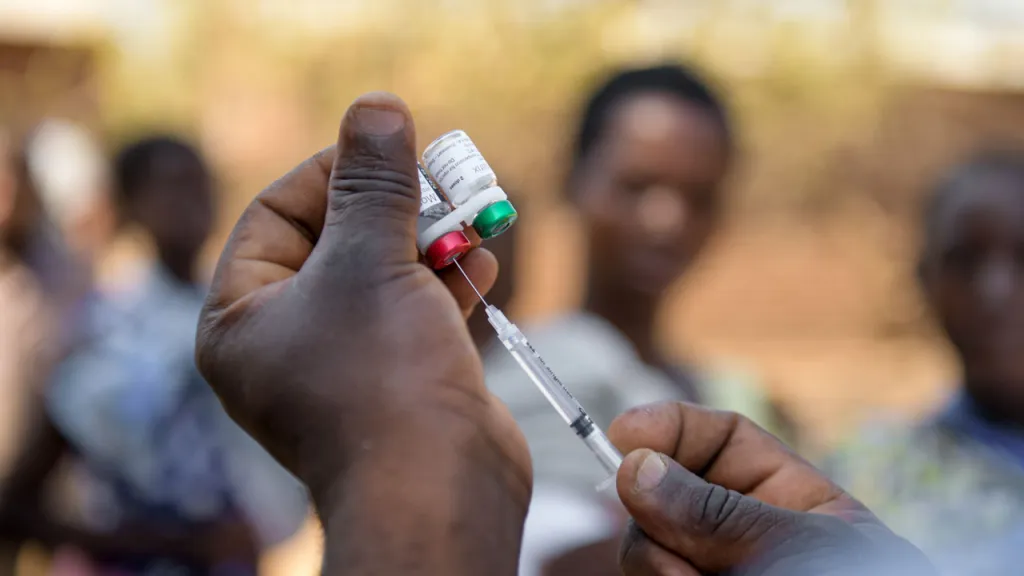
While the introduction of the vaccine is a milestone and life-saving measure, Willis Akhwale from End Malaria Council Kenya emphasizes the modest efficacy rate not being a “silver bullet.”
Medical professionals view the vaccine as an additional tool in the ongoing battle against malaria. It complements existing preventive measures like mosquito nets and antimalarial tablets. According to a UK-led study, employing all three interventions concurrently potentially provides children with 90% protection against malaria.
Cameroonian doctor Shalom Ndoula is a key figure in overseeing the vaccine rollout in his country. He sees the combined approach as a strategy to reduce malaria cases and deaths and, in the end, eliminate the disease.
The development of the RTS,S vaccine by British pharmaceutical company GSK spanned 30 years of research. WHO approved the vaccine, and lauds its introduction in Cameroon as a historic moment in the global campaign against the mosquito-borne disease.
Earlier this month, Cape Verde became the first sub-Saharan African country in 50 years to attain malaria-free status as declared by WHO.
The Safety and Effectiveness of the Malaria Vaccine in Cameroon
As Cameroon Rolls out a World-First Malaria Vaccine, concerns about the safety and effectiveness of the vaccine have led to fears and doubts among some Cameroonians. Wilfred Fon Mbacham, a Cameroonian king and a professor of public health biotechnology specializing in malaria, countered claims that Cameroonians remain guinea pigs. Instead, he emphasised the need for scientists to educate the public about the vaccine’s nature and benefits to alleviate fears.
Daniele Ekoto, a vaccination official at the launch, reassured mothers about the safety, effectiveness, and free nature of the vaccine for children. In 2021, Africa accounted for 95% of global malaria cases and approximately 96% of related deaths. Cameroon alone records about six million malaria cases annually. Which results in 4,000 deaths in health facilities, primarily affecting children under five.
The vaccination program targets six-month-old children in the 42 districts with the highest rates of morbidity and mortality. The children receive four doses until the age of two. Twenty other countries, including Burkina Faso, Liberia, Niger, and Sierra Leone, plan to implement the program this year (Gavi). However, the demand for doses surpasses the available supply of approximately 18 million before 2025. Thus falls short of the recommended doses for approved countries.
A second vaccine, R21, developed by Oxford University, increases the available doses in future addressing the shortages. The Serum Institute of India plans to manufacture 100 million doses per year. However, it pends successful regulatory approvals following the WHO recommendation for use last year.